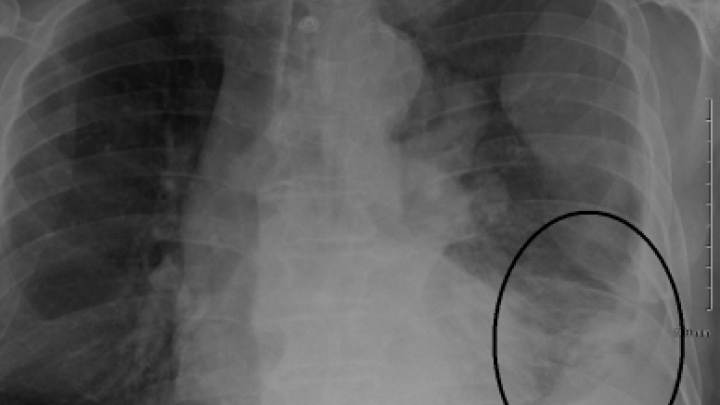
Clinical Cancer Trials: At Times an Overlooked Option
Clinical trials offer treatment options that are not otherwise available to cancer patients. Investigational cancer drugs and drug combinations may represent the best hope for certain patients, but they can only be given in the context of a clinical study.
Although many patients could personally benefit from cancer trials, many do not enroll. According to a 2010 National Institute of Health workshop on U.S. clinical research, only 3% of adult cancer patients enter trials. In a recent review of over 500 National Cancer Institute trials, almost 40% were unable to enroll a sufficient number of patients. This lack of participation is more than just a problem for medical research. It is a problem for individual patients. For some patients, the new therapy may be their best chance. Lack of awareness and common misconceptions, however, are often a barrier.
One of the most misunderstood aspects of trials is the use of placebos. Cancer patients shy away because they are afraid of receiving a placebo instead of therapy. It cannot be overemphasized; this will not happen in a cancer trial. Cancer patients always receive, at the minimum, the standard therapy regimen. If the medication to be tested is an adjunct, then it is possible a patient may receive a placebo for the adjunct alone. However, the patient will always receive the standard treatment.
Loss of control is another reason that patients avoid cancer trials. Although patients may fear being stuck in a trial that is not working for them, enrollment is completely voluntary. A patient can withdraw from a trial at any point. There is no requirement that you stay in the trial if you decide that it is no longer right for you.
Randomization is another source of concern. Phase III studies may divide patients into groups. One group will receive the investigational treatment while the other is the control group. As the patient cannot choose the group, there is no guarantee of receiving the new drug. However, trials are stopped early if a clear benefit is seen, and control groups may still receive the investigational drug when a study is stopped early. The patient's health is of prime importance in clinical cancer studies, and doctors will not allow a trial to continue once clear differences are seen.
Travel and location are also cited, but clinical trials are actually run throughout the country. Although some studies are still held solely in the specialty research centers, many are not. It is possible to find ongoing studies held in nearby medical centers.
Patients may avoid trials because of insurance issues. Insurers do balk at covering trial drugs, and some even refuse to cover the trial's lab procedures. However, the physicians and research coordinators are well-versed in dealing with these problems. They can often work with your insurer. In addition, the trial sponsor may be willing to cover some of the costs that insurers refuse. Before declining study participation, determine what your out-of-pocket expenses will actually be.
The American Cancer Society, National Cancer Institute (NCI) and National Institutes of Health (NIH) all provide detailed material on clinical studies. The NCI and NIH maintain online lists of active trials, and both the American Cancer Society and Centerwatch provide online matching services to help patients find appropriate ongoing trials. There is a wealth of online information available that can help to alleviate study concerns.
Cancer patients need to assess all of their options, including clinical trials. These studies are not only for the benefit of future patients, they can be lifesaving for the patients that are enrolled.